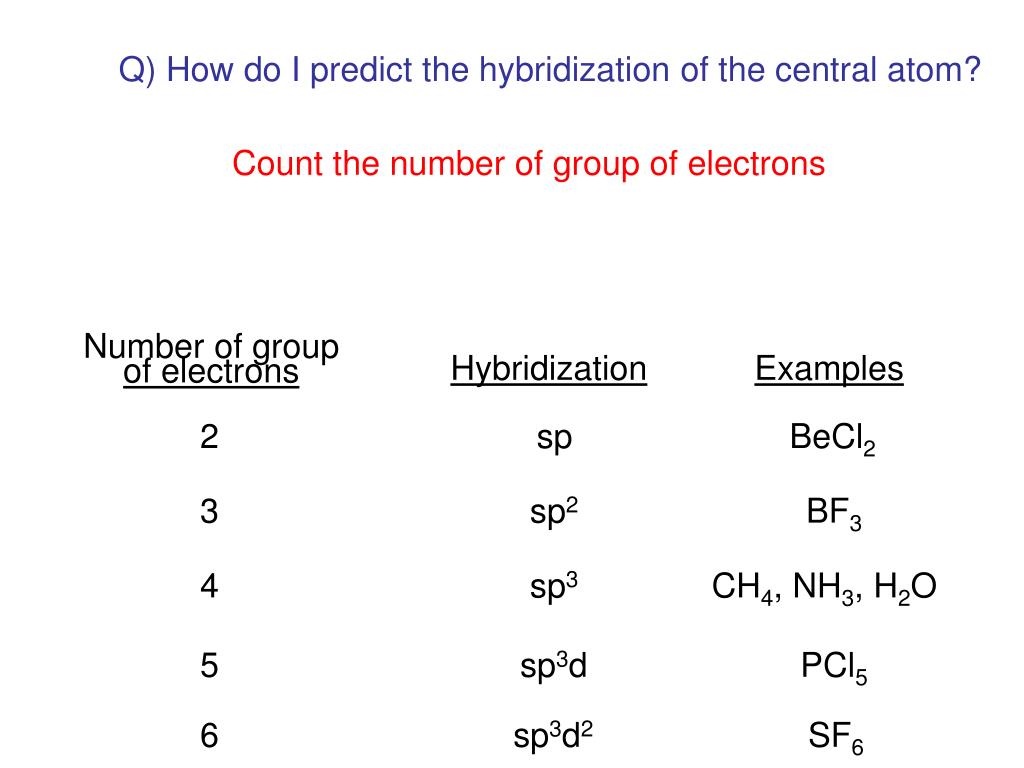
PPT - Chemical Bonding II: Molecular Geometry and Hybridization of Atomic Orbitals PowerPoint Presentation - ID:5587869
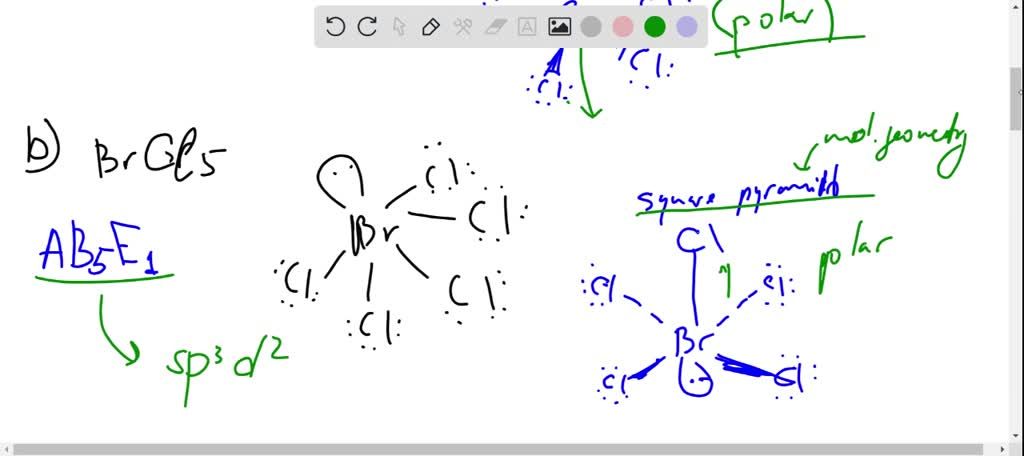
SOLVED: For each of the following molecules: (i) draw the correct Lewis structure; (ii) determine the molecular geometry and the type of hybridization on the central atom, and (iii) predict whether the
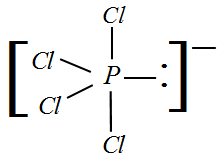
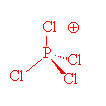
What are the shapes of $PC{l_4}^ + $ , $PC{l_4}^ - $ and $AsC{l_5}$ ?A. Square planar, tetrahedral and see-sawB. Tetrahedral, see-saw and trigonal bipyramidalC. Tetrahedral, square planar and pentagonal bipyramidalD. Trigonal
Lecture 18 - Covalent Bonding Lecture 18 - Introduction Lecture 18 - Valence Bond Theory Lecture 18 - Valence Bond Theory Lectur

The shapes of PCl^+_4,, P{ Cl }^-_4 and AsCl_5 are respectively:tetrahedral, see-saw and trigonal bipyramidalsquare planar, tetrahedral and see-sawtetrahedral, square planar and pentagonal bipyramidaltrigonal bipyramidal, tetrahedral and square pyramidal

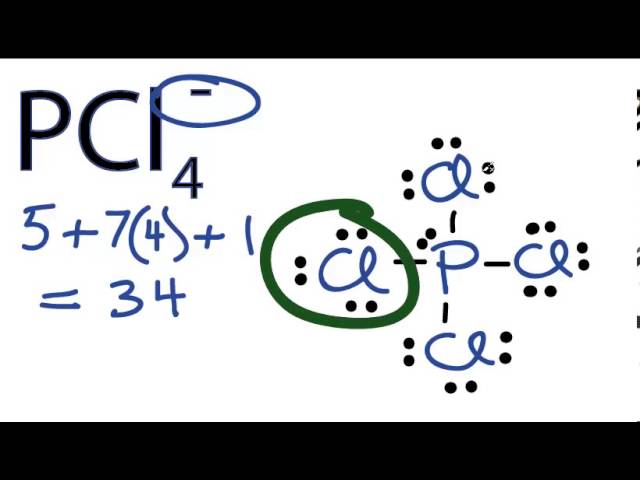
Which of the following has a bond angle of approximately 120 deg? a) ClF3 b) SbBr6- c) PCl4- d) BeCl2 | Homework.Study.com

PCl4+ (Phosphorus tetrachloryl ion) Molecular Geometry, Bond Angles (and Electron Geometry) - YouTube





_1.jpg)